The pharmaceutical industry increasingly needs new methodological tools that enable it to synthesize active ingredients in a more specific manner, building new couplings more efficiently and reducing purification steps while carrying them out in milder and more sustainable conditions. Hypervalent iodine chemistry is increasingly present in the field of organic synthesis. Among the various applications, iodine derivatives with a high oxidation level stand out for their role as oxidizing agents and for transferring electrophilic groups. In recent years, however, several innovative reactivity patterns of λ3-iodane reagents have emerged, which offer new opportunities for the development of synthetic methodologies.
New Synthetic Methodologies Based on Hypervalent Halogens

Within this context, Dr Wei Wen Chen recently conducted his doctoral thesis at IQS, in which he developed new synthetic methodologies based on hypervalent halogen chemistry as a tool to explore new transformations and strategies for designing structures with high selectivity. Entitled The hypervalent halogen chemistry as enabling tool for new C-H coupling reactivity and structure design, he carried out his thesis within the Chemical Reactions for Innovative SOLutions (CRISOL) research group, and was jointly supervised Dr Ana B. Cuenca, with the Department of Organic and Pharmaceutical Chemistry at IQS, and Dr Alexandr Shafir, with the Advanced Chemistry Institute of Catalonia (IQAC).
In his thesis, Dr Chen explored the reactivity of organic species containing hypervalent halogens with various organometallic compounds, focusing in particular on the use of organometalloids based on boron and silicon.
New C-H couplings
The modification of the carbon-hydrogen (C-H) coupling, the most abundant in organic molecules, is a very complicated task using classical methodologies due to extreme reaction conditions, the use of transition metals, and low selectivity. This thesis has provided new reactivities starting from PhIX2 type species, highlighting the ability of certain transient iodine(III) intermediates to facilitate the transfer of a group to the iodoarene nucleus, resulting in a C-H coupling without the need for a metal catalyst, selectively, and under very mild conditions. Specifically, the (diacetoxy)iodine group acts as a directing element, facilitating the selective introduction of certain organic fragments in the ortho or para positions with respect to the iodine atom of an aromatic ring.
Among the achievements of this transformation is the high efficiency observed in the iodonium-Claisen coupling of hypervalent iodine precursors with allylsilane derivatives, which has facilitated the formation of more than 50 examples of ortho-allylated iodoarene compounds. The synthetic potential of these ortho–iodoallylarenes has been demonstrated in a variety of transformations, ranging from double bond migration and olefination-type reactions to the synthesis of relevant compounds, such as versatile synthetic intermediates or derivatives of drug-like molecules.
Related publication
Wei W. Chen et al, Iodane-Guided ortho C−H Allylation, Angewandte Chemie International Edition, 2020, 59, 45, 20201-20207
Gem-dimethaloids as versatile nucleophiles
In another section of his research, Dr Chen expanded the synthetic panorama of the versatile benzylic compounds gem-α,α-dimethaloids based on boron/silicon, ranging from the generation of these species to their applications in reactivity. The inherent differences in reactivity between the two carbon-metalloid couplings have been exploited to generate two orthogonal methodologies that allow the C-Si and C-B fragments to be chemoselectively modified. On the one hand, under the influence of hypervalent iodoarene species, chemoselective arylation of the C-Si fragment has been achieved. On the other hand, it has been shown that the C-B fragment of these gem-dimetallic derivatives undergoes efficient and chemoselective arylation of the Suzuki–Miyaura type with various Ar–Cl.
The methodology employed provides a solid basis for the development of additional methods aimed at the modular and iterative construction of multi-substituted carbon centres, a task of great importance in synthetic chemistry today.
Related publication
Related to this part of the research, the group has published an article that is among the most popular Organic Chemistry publications by the Royal Society of Chemistry in 2021.
Wei W. Chen et al, Exploring the benzylic gem-C(sp3)-boron-silicon and boron-tin centres as a synthetic platform, Chemical Science, 2021, 12, 10514-10521
Application of diaryl-iodonium salts
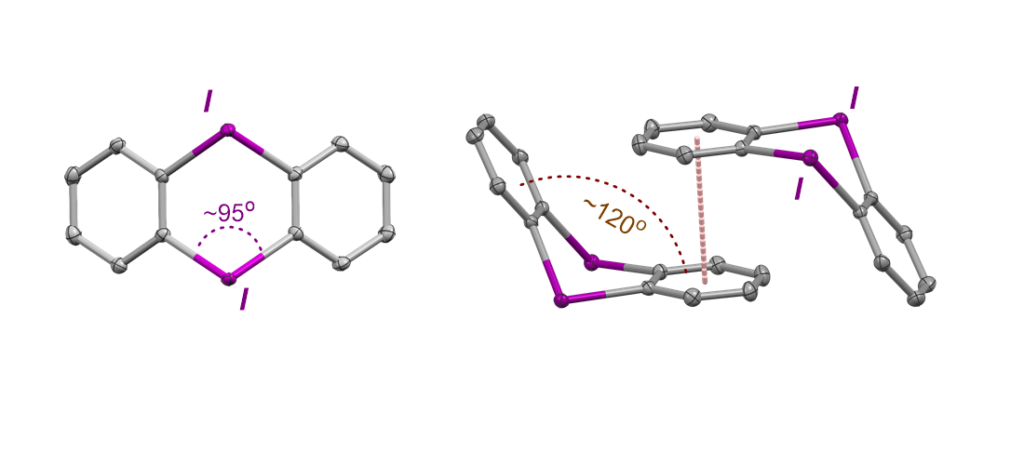
Finally, thanks to the research conducted by Chen, it has been possible to expand the structural space of diaryliodonium salts with a new family of these cyclic structures that contain two hypervalent halogens in a ring, which did not exist in the literature until now. These new rigid architectures represent an interesting platform for generating new “angular bar” type molecular geometries thanks to the nearly 90° angle characteristic of the C−I−C unit. In addition, it has been possible to demonstrate that the pairs of iodine(III) acid vectors can be adjusted by modifying the aromatic ring, thus opening the exploration of new methods of chelator and bridge binding.
Related publication
Wei W. Chen et al, Cyclic Homo- and Heterohalogen di-λ3-diarylhalonium Structures, Journal of the American Chemical Society. 2023, 145, 25, 13796–13804

This thesis has received funding under the project BISiBonds – New synthesis models, within the 2020 State Plan for R&D&i Projects with the Ministry of Science and Innovation/State Innovation Agency.