The Pharmaceutical Chemistry Group (GQF) is an interdepartmental research group created as a result of the synergy between members of different laboratories at the IQS School of Engineering – Ramon Llull University (URL): Molecular Design Laboratory, Synthesis Laboratory, Spectroscopy Laboratory, Tissue Engineering Laboratory, and Thermal Analysis Laboratory. Their joint objective is to develop new drugs capable of improving people’s quality of life, in line with Sustainable Development Goal (SDG) 3 – Good Health and Wellbeing. The group has renewed its recognition as a 2021 SGR Consolidated Research Group by the Agency for the Management of University and Research Grants (AGAUR) within the Government of Catalonia. The group is currently led by Dr Roger Estrada Tejedor.
Within the range of solutions offered by the IQS Tech Transfer division in Pharmacy and Cosmetics, the experience of the different members of the GQF and the variety of disciplines it covers enable it to address nearly all stages of the development of a drug candidate. In this sense, the GQF constitutes a unique group within the field of research in Catalonia.
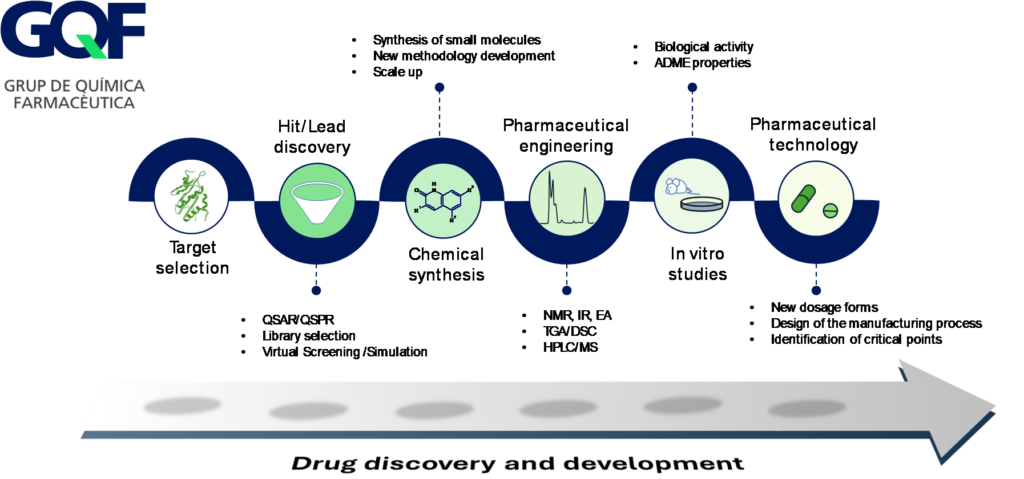
Capacities of the GQF Group
The GQF’s main lines of research are focused on Medical and Pharmaceutical Chemistry, including the design, synthesis, and evaluation of the activity of new drug candidates, pharmaceutical and cosmetic formulation, molecular biology, and the production of new compounds of interest in the areas of biomedicine and health sciences.
The GQF is organized into three areas of expertise that constitute the basic structural units and the different laboratories where all the research activity or contracts with companies are carried out.
Medical Chemistry Area
This unit boasts the Molecular Design Laboratory, with the capacity to carry out modelling and simulation studies of (bio)molecular systems to design drugs, select compounds, design new molecules with properties or activities of interest (QSAR models), generate prediction models (using machine learning and artificial intelligence tools), and solve computational chemistry problems.
The Synthesis Laboratory features the ability to synthesize organic molecules, ensure their characterization, and offer advice on the design of routes for obtaining and/or scaling synthesis processes, whether for APIs, impurities, prodrugs, or more.
The Tissue Engineering Laboratory offers the possibility of evaluating the effect of synthesized compounds on the diseases it studies compared to biological models, both at the 2D and 3D levels, and develops bioengineering and biomedicine platforms. This laboratory also studies the improvement of ADME properties, helping to find the structural points most likely to undergo the metabolism process, which makes it possible to modify the original structure in an iterative process to avoid rapid metabolism and thus avoid low bioavailability.
Pharmaceutical Engineering Area
This unit is home to different analysis and control laboratories, such as Spectroscopy (NMR, IR, Elemental Analysis, Polarimetry), which facilitates the identification and characterization of the different synthesized compounds. The use of HPLC/MS is essential for maintaining purity control during the obtaining processes and the final products, and for their identification. The Thermal Analysis and Calorimetry laboratory makes it possible to elucidate pharmaceutical forms (crystallinity, existence of co-solvents, etc.), as well as guarantee issues related to process safety.
Pharmaceutical Technology Area
The objective of new drug development is administering them to patients and their delivery in the final dosage form is essential to facilitate the administration of the dose defined in the clinical trials, ensuring the efficacy of the drug. Using Pharmaceutical Technology tools, solid or liquid dosage forms can be developed for oral administration or semi-solid dosage forms for topical administration.
The design of a drug includes the design of the corresponding optimized manufacturing process and the identification and control of possible critical points. With regard to topical forms of action, it is interesting to apply artificial biocompatible membranes to evaluate the performance of the product (pharmaceutical or cosmetic).
GQF lines of experience
The capabilities of the GQF group can be adapted to multiple areas depending on the needs of each project and client. Currently, the group’s lines of work focus on the following topics, among others:
- Studying new therapeutic strategies to treat rare muscular dystrophies.
- Developing new drug candidates against diffuse large B-cell lymphoma (DLBCL), pancreatic cancer, and breast cancer.
- Designing and developing new antiviral compounds as phytosanitary products.
- Developing methodologies for analysing products under investigation.
Noteworthy collaborations
Over the past few years, the GQF has collaborated with various research groups (Spanish and international) to design new compounds for the inhibition of pathogenic RNAs with researchers from the National Research Council of Italy, the Université Côte d’Azur, and the University of Valencia. In other areas within the field of oncology, the group has collaborated with the Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Oncology Institute (VHIO), and the Josep Carreres Leukaemia Research Institute.
We can also highlight other outstanding collaborations with companies such as GSK to study the development of antimalarial compounds using machine learning methods, or with Pangaea Oncology to develop new compounds against lung cancer.
The GQF has collaborated, and continues to collaborate, with various companies in the biopharmaceutical sector to develop and synthesize new chemical entities and to optimize drug candidates, in addition to improving the synthesis process in APIs, minimizing the formation of impurities, and synthesizing and characterizing these impurities during the process of obtaining an API.