MNK Inhibitor: Overcoming Therapy Resistance and Immune Evasion
Investigador principal
Grupo de investigación

Fuente de financiación:
Gobierno de España
Periodo:
01/03/2023 a 30/03/2024
Estado del proyecto:
FINALIZADO
Financiación:
119,996 €
Development of a novel MNK inhibitor to treat breast and prostate cancer
The acquisition of therapy resistance remains a main cause of therapeutic failure in cancer treatment.6 Immunotherapy has opened a new avenue in oncology, but is only effective in a small fraction of patients.
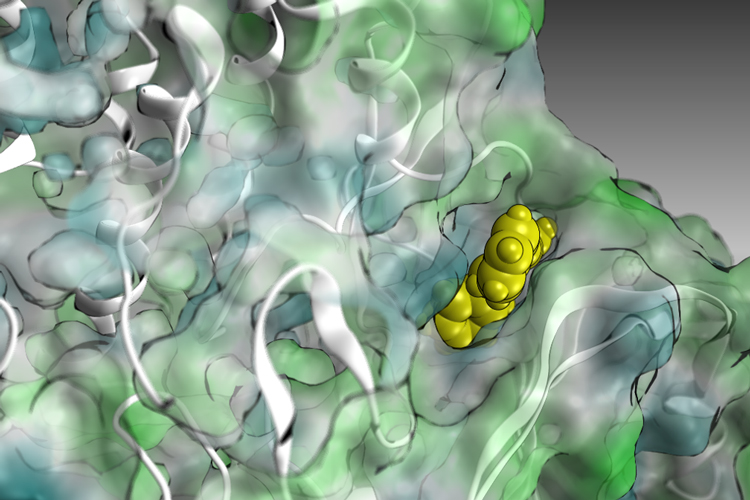
In search of long-term cure, immunotherapies, mainly targeting immune checkpoint inhibitors (e.g. PD1), have provided new hope for many cancer patients. However, treatments are only effective in a small fraction of patients, which currently limits the broad application of immunotherapies. Currently, 43.63% of patients are defined as eligible for immunotherapy across different cancers but the estimated rates of response to checkpoint inhibitor drugs is only 12.46%.Thus, therapy resistance and immune evasion represent a current unmet clinical need in cancer cure Therapy resistance and immune evasion critically rely on the activation of stress response pathways.11 Those pathways activate the kinases MNK1/2 which uniquely regulate the translation initiation factor eIF4E at S209.4,12We aim at exploiting novel therapeutic strategies, combining the MNK inhibitor EB1 with standard-of-care therapies, to achieve a superior therapeutic index that translates into a genuine impact on patients.The ultimate goal of our project is to provide a new therapeutic opportunity ready to enter IND-enabling studies and future clinical trials. We propose here to combine our technology with FDA approved therapies, which have been proven to be effective for selected patient groups, but are ineffective for others with similar clinical characteristics.Our objectives are:1) Single cell sequencing to clarify mode of action of EB12) Preclinical assessment of MNK inhibition in vivo3) Pharmacological Validation and Optimization of EB1 as clinical drug candidate4) Clinical identification of treatment options for future patient stratification
Colaboradores / Entidad financiadora

VHIR

CicBiogune